购物车
- 全部删除
 您的购物车当前为空
您的购物车当前为空

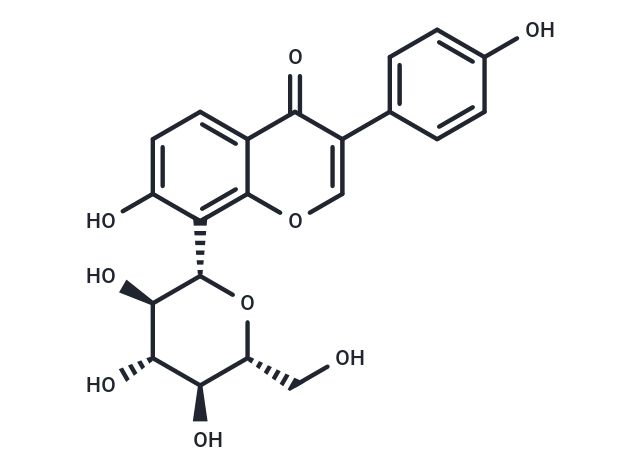
Puerarin (Kakonein) 是从葛根中提取的一种异黄酮,是一种5-HT2C 受体拮抗剂。

为众多的药物研发团队赋能,
让新药发现更简单!
Puerarin (Kakonein) 是从葛根中提取的一种异黄酮,是一种5-HT2C 受体拮抗剂。
| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5 mg | ¥ 247 | 现货 | |
| 10 mg | ¥ 345 | 现货 | |
| 25 mg | ¥ 488 | 现货 | |
| 50 mg | ¥ 638 | 现货 | |
| 100 mg | ¥ 828 | 现货 | |
| 500 mg | ¥ 1,980 | 现货 | |
| 1 mL x 10 mM (in DMSO) | ¥ 382 | 现货 |
| 产品描述 | Puerarin (Kakonein), also known as Kakonein, is a member of the class of compounds known as isoflavonoid C-glycosides. It is a 5-HT2C receptor antagonist. |
| 体外活性 | 给高胆固醇饮食的大鼠每天口服300 mg/kg Puerarin,显著降低高胆固醇饮食引起的血清和肝脏中总胆固醇含量的增高. |
| 体内活性 | Puerarin在25 μM下剂量依赖性减少HT-29细胞的生长,伴随bax的增加,以及c-myc和bcl-2的减少。 |
| 激酶实验 | STAT3-dependent dual-luciferase assay: HCT-116 cells are transiently transfected with reporter plasmid having the STAT3-binding element for regulating luciferase assay. Cells are treated with Cryptotanshinone for 24 hours at a concentration range of 0.2 to 50 μM. After treatment, cells are harvested in 20 μL of passive lysis buffer and luciferase activity is evaluated by the Dual Luciferase Reporter Assay kit on Wallac Victor2. The concentration of Cryptotanshinone that inhibits the luciferase activity by 50% represents IC50 value. |
| 细胞实验 | RAW264.7 cells are maintained at subconfluence in 95% air and 5% CO2 humidified atmosphere maintained at 37°C. The medium used for routine subculture is Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum, penicillin (100 units/mL) and streptomycin (100 μg/ mL). An MTT assay is used to measure the viability of the cells after treatment with puerarin. After the supernatants are removed for nitrite determination, cells are incubated at 37°C with MTT (0.05 mg/mL) for 4 h, and the optical density is measured at 540 nm. The concentrations of puerarin are10, 20, 40 and 100 μM[1]. |
| 别名 | 葛根素, Kakonein |
| 分子量 | 416.38 |
| 分子式 | C21H20O9 |
| CAS No. | 3681-99-0 |
| Smiles | OC=1C(=C2C(C(=O)C(=CO2)C3=CC=C(O)C=C3)=CC1)[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O |
| 密度 | 1.642 g/cm3 |
| 存储 | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. | |||||||||||||||||||||||||||||||||||
| 溶解度信息 | DMSO: 50 mg/mL (120.08 mM), Sonication is recommended. Ethanol: < 1 mg/mL (insoluble or slightly soluble) H2O: < 1 mg/mL (insoluble or slightly soluble) | |||||||||||||||||||||||||||||||||||
溶液配制表 | ||||||||||||||||||||||||||||||||||||
DMSO
| ||||||||||||||||||||||||||||||||||||
| 中药材名称 | 中药材拉丁名 | 性 | 味 | 归经 |
|---|---|---|---|---|
| 柴胡 | Bupleurum scorzonerifolium Willd., Bupleurum chinense DC. | 微寒 | 辛, 苦 | 肝, 胆, 肺 |
| 大枣 | Ziziphus jujuba Mill. | 温 | 甘 | 脾, 胃, 心 |
| 粉葛 | Pueraria thomsonii Benth | 凉 | 甘, 辛 | 脾, 胃 |
| 甘葛藤根 | Pueraria thomsonii Benth. | 凉 | 甘, 辛 | 脾, 胃 |
| 中成药名称 | 处方组成 | 中成药类型 |
|---|---|---|
| 安神补脑胶囊 | 鹿茸,制何首乌,淫羊藿,干姜,甘草,大枣,维生素B1 | 安神药 |
| 表热清胶囊 | 柘树根,石膏,南板蓝根,金银花,柴胡,黄芩,甘草 | 清热药 |
| 安神补脑片 | 鹿茸,制何首乌,淫羊藿,干姜,甘草,大枣,维生素B1 | 安神药 |
| 半夏和胃颗粒 | 半夏(姜制),黄芩,干姜,黄连,党参,炙甘草,大枣 | 化痰、止咳、平喘药 |
| 阿胶三宝颗粒 | 阿胶,大枣,黄芪 | 扶正药 |
| 百咳宁颗粒 | 柴胡,荆芥,半夏,茯苓,贝母 | 化痰、止咳、平喘药 |
| 鳖甲煎丸 | 鳖甲胶,阿胶,蜂房(炒),鼠妇虫,土鳖虫(炒),蜣螂,硝石(精制),柴胡,黄芩,半夏(制),党参,干姜,厚朴(姜制),桂枝,白芍(炒),射干,桃仁,牡丹皮,大黄,凌霄花,葶苈子,石韦,瞿麦 | 祛瘀药 |
| 葆宫止血颗粒 | 牡蛎,白芍,侧柏叶,地黄,金樱子,柴胡,三七,椿皮,仙鹤草,大青叶 | 理血药 |
| 安眠补脑颗粒 | 制何首乌,远志(制),柏子仁,枸杞子,麦冬,五味子(醋制),桑椹,大枣,红参,甘草(炙) | 安神药 |
| 安神补脑分散片 | 鹿茸,制何首乌,淫羊藿,干姜,甘草,大枣,维生素B1 | 安神药 |
| 宝宝乐咀嚼片 | 白芍,黄芪(蜜炙),大枣,山楂(炒),麦芽(炒),桂枝,干姜,六神曲(焦) | 扶正药 |
| 白草香解郁安神胶囊 | 夏枯草,白芍,合欢花,酸枣仁炒,柴胡,香附,地黄,五味子,首乌藤 | 理气药 |
| 表虚感冒胶囊 | 桂枝,葛根,白芍,炒苦杏仁,生姜,大枣 | 清热药 |
| 鼻渊舒胶囊 | 辛夷,苍耳子,栀子,黄芩,黄芪,川芎,柴胡,细辛,薄荷,川木通,茯苓,白芷,桔梗 | 清热药 |
| 安尔眠胶囊 | 丹参(切片),首乌藤,大枣 | 安神药 |
| 安眠补脑口服液 | 制何首乌,远志(制),柏子仁,枸杞子,麦冬,五味子(醋制),桑椹,大枣,红参甘草(炙) | 安神药 |
| 安乐胶囊 | 柴胡,当归,川芎,茯苓,钩藤,首乌藤,白术(炒),甘草 | 安神药 |
| 表热清颗粒 | 柘树根,南板蓝根,石膏,金银花,柴胡,黄芩,甘草 | 清热药 |
| 安尔眠颗粒 | 丹参(切片),首乌藤,大枣 | 安神药 |
| 宝宝乐(小儿健脾颗粒) | 白芍,黄芪(蜜炙),大枣,桂枝,干姜,山楂(炒),六神曲(焦),麦芽(炒) | 扶正药 |
| 方剂名称 | 处方组成 | 剂型 | 处方来源 |
|---|---|---|---|
| 五积交加散 | 羌活,苍术,防风,枳壳,陈皮,柴胡,当归,川芎,独活,白芷,半夏,麻黄,桔梗,茯苓,厚朴,桂枝,甘草 | 散剂 | 《寿世保元》卷七。 |
| 五味子散10 | 五味子,酸枣仁,人参,白术,炙甘草,黄芪,诃子,柴胡 | 散剂 | 《圣惠》卷二十七。 |
| 六一顺气汤 | 大黄,枳实,黄芪,厚朴,甘草,柴胡,芒硝,芍药 | 汤剂 | 《伤寒六书》卷三。 |
| 五果茶 | 核桃,银杏,大枣,栗 | 茶剂 | 《济众新编》卷七。 |
| 五味子汤3 | 五味子,甘草,紫菀,肉桂,附子,麻黄,干姜,川芎,细辛,大枣 | 汤剂 | 《外台》卷十六引《删繁方》。 |
| 五味子散12 | 五味子,细辛,贝母,柴胡,桑白皮,射干,陈皮,炙甘草 | 散剂 | 《圣惠》卷十四。 |
| 五味败毒散 | 羌活,独活,前胡,柴胡,枳壳,桔梗,甘草,人参,茯苓,川芎,大黄,苍术 | 散剂 | 《赤水玄珠》卷十一。 |
| 六合汤 | 当归,大黄,川芎,熟地黄,白芍,柴胡 | 汤剂 | 《水类钤方》卷二十。 |
| 五痫汤 | 大黄,钩藤,蜂房,麻黄,柴胡,栀子,知母,芍药,升麻,蚱蝉,石膏,蛇蜕,杏仁 | 汤剂 | 《幼幼新书》卷十一引《婴孺方》。 |
| 五味子散1 | 五味子,陈皮,紫菀,贝母,杏仁,麻黄,麦冬,甘草,赤茯苓,柴胡 | 散剂 | 《圣惠》卷四十二。 |
| 五果膏 | 龙眼肉,大枣,核桃,莲子,榧子 | 膏剂 | 《医钞类编》卷六。 |
| 五积交加散1 | 苍术,陈皮,芍药,白芷,当归,肉桂,半夏,厚朴,干姜,麻黄,柴胡,川芎,前胡,甘草,人参,桔梗,羌活,独活,茯苓,枳壳,薄荷,木瓜 | 散剂 | 《寿世保元》卷五。 |
| 五味子汤2 | 五味子,蒺藜,麻黄,桑白皮,白石脂,杏仁,百合,贝母,款冬花,枳壳,紫菀,柴胡,旋覆花,桂枝 | 汤剂 | 《圣济总录》卷六十五。 |
| 五苓平胃汤 | 柴胡,黄芩,苍术,半夏,甘草,白术,陈皮,茯苓,厚朴,猪苓,泽泻,桂枝 | 汤剂 | 《嵩崖尊生》卷九。 |
| 五味子汤26 | 五味子,大枣,桑白皮,藁本,钟乳,款冬花,薄荷 | 汤剂 | 《外台》卷十引《广济方》。 |
| 五柴胡饮 | 柴胡,当归,熟地黄,白术,芍药,炙甘草,陈皮 | 汤剂 | 《景岳全书》卷五十一。 |
| 五味汤2 | 五味子,黄芩,柴胡,芒硝,麦冬,石膏,黄连,甘草,当归,大黄 | 汤剂 | 《幼幼新书》卷十四引《婴孺方》。 |
| 六甲散 | 沉香,槟榔,甘草,木香,龙骨,人参,茯苓,柴胡,青皮,甘松,半夏,藿香,生地黄,肉桂,陈皮,当归,鳖甲 | 散剂 | 《普济方》卷八十五。 |
| 五味竹叶汤 | 竹叶,五味子,前胡,当归,干地黄,人参,小麦,黄芪,黄芩,麦冬,生姜,炙甘草,升麻,大枣,肉桂 | 汤剂 | 《鬼遗》卷三。 |
| 五味子汤8 | 五味子,肉桂,炙甘草,细辛,干姜,紫菀,大枣,麻黄 | 汤剂 | 《外台》卷九引《深师方》。 |
以上为“体内实验配液计算器”的使用方法举例,并不是具体某个化合物的推荐配制方式,请根据您的实验动物和给药方式选择适当的溶解方案。
评论内容