Powder: -20°C for 3 years | In solvent: -80°C for 1 year
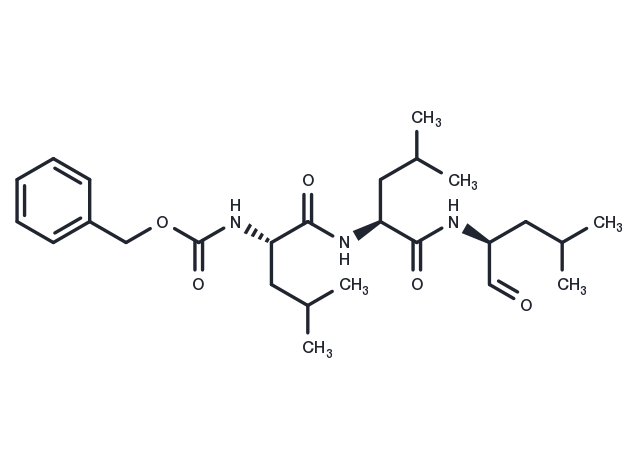
MG-132 (Z-Leu-Leu-Leu-al) 是一种 26S 蛋白酶体抑制剂 (IC50=100 nM),具有细胞渗透性、可逆性。MG-132 可作为自噬激活剂,可诱导凋亡。
| 规格 | 价格/CNY | 折后价 | 货期 | 数量 |
|---|---|---|---|---|
| 1 mg | ¥ 137 | ¥ 68 | 现货 | |
| 5 mg | ¥ 276 | ¥ 138 | 现货 | |
| 10 mg | ¥ 382 | ¥ 191 | 现货 | |
| 25 mg | ¥ 828 | ¥ 414 | 现货 | |
| 50 mg | ¥ 1,280 | ¥ 640 | 现货 | |
| 100 mg | ¥ 1,850 | ¥ 925 | 现货 | |
| 200 mg | ¥ 2,580 | ¥ 1,290 | 现货 | |
| 500 mg | ¥ 4,330 | ¥ 2,165 | 现货 | |
| 1 mL * 10 mM (in DMSO) | ¥ 353 | ¥ 176 | 现货 |
| 产品描述 | MG-132 (Z-Leu-Leu-Leu-al) is a 26S proteasome inhibitor (IC50=100 nM) that is cell-permeable and reversible. MG-132 acts as an autophagy activator and also induces apoptosis. |
| 靶点活性 | Calpain:1.2 μM (cell free), 20S proteasome:100 nM (cell free) |
| 体外活性 |
方法:人宫颈癌细胞 HeLa 用 MG-132 (0.5-30 μM) 处理 24 h,使用 MTT 方法检测细胞生长抑制情况。 结果:MG-132 剂量依赖性地抑制 HeLa 细胞生长,IC50 约为 5 μM。[1] 方法:人间皮瘤细胞 NCI-H2452 用 MG-132 (0.25-2 μM) 处理 36 h,使用 Western Blot 方法检测靶点蛋白表达水平。 结果:MG-132 处理诱导 NCI-H2052 细胞中 caspases 3、caspases 7、Bid 和 PARP 的切割,诱导 caspase 依赖性凋亡。[2] 方法:人类黑色素瘤细胞 MeWo 用 MG-132 (0.01-1 μM) 处理 24 h,使用 Flow Cytometry 方法分析细胞周期情况。 结果:MG-132 诱导 MeWo 细胞的细胞周期阻滞在 G2 期。[3] |
| 体内活性 |
方法:为检测体内抗肿瘤活性,将 MG-132 (1 mg/kg) 静脉注射给携带人宫颈癌肿瘤 HeLa、CaSki 或 C33A 的 C.B‐17/lcr‐scid/scidJcl 小鼠,每周两次,持续四周。 结果:MG-132 治疗显著抑制人宫颈癌肿瘤的生长,表明在体内具有抗肿瘤活性。[4] 方法:为研究 MG-132 长期治疗对心肌肥大的影响及其相关分子机制,将 MG-132 (0.1 mg/kg) 腹腔注射给具有腹主动脉束带(AAB)的大鼠,每天一次,持续八周。 结果:MG-132 治疗显著减弱了 AAB 大鼠的左心室肌细胞面积、左心室重量/体重和肺重量/体重比,降低了左心室舒张直径和壁厚,并增加了缩短分数。MG-132 治疗可显著逆转 AAB 大鼠 ERK1/2 和 JNK1 磷酸化水平的升高。[5] |
| 激酶实验 | Inhibitory activities of ZLLa1 and ZLLLal against m-calpain and 20S proteasome were measured by previously described methods.For the m-calpain inhibitory assay,the 0.5 ml reaction mixture contained 0.24% alkali-denatured casein,28 mM 2-mercaptoethanol,0.94 unit of m-calpain,ZLLal or ZLLLal,6 mM CaCl2,and 0.1M Tris-HC1 (pH 7.5).The reaction was started by the addition of m-calpain solution and stopped by the addition of 0.5 ml of 10% trichloroacetic acid after incubation at 30℃ for 15 min.After centrifugation at 1,300×g for 10 min,the absorbance of the supernatant at 280 nm was measured.The reaction mixture for the 20S proteasome inhibitory assay contained 0.1 M Tris-acetate,pH 7.0,20S proteasome,ZLLa1 or ZLLLal,and 25 μM substrate dissolved in dimethyl sulfoxide in a final volume of 1 ml.After incubation at 37℃ for 15 min,the reaction was stopped by the addition of 0.1 ml of 10% SDS and 0.9 ml of 0.1 M Tris-acetate,pH 9.0.The fluorescence of the reaction products was measured.To determine the IC50s against m-calpain and 20S proteasome,various concentrations of the synthetic peptide aldehydes were included in the assay mixture [1]. |
| 细胞实验 | The effect of MG132 on HeLa cell growth was determined by trypan blue exclusion cell counting or measuring MTT dye absorbance of living cells as previously described. In brief, cells (5x10^5 cells per well) were seeded in 24-well plates for cell counting, and cells (5x10^4 cells per well) were seeded in 96-well microtiter plates for the MTT assay. After exposure to indicated amounts of MG132 for 24 h, cells in 24-well plates or 96-well plates were collected with trypsin digestion for trypan blue exclusion cell counting or were used for the MTT assay. Twenty microliters of MTT solution (2 mg/ml in PBS) was added to each well of 96-well plates. The plates were again incubated for 4 h at 37?C. MTT solution in the medium was aspirated off and 200 μl of DMSO was added to each well to solubilize the formazan crystals formed in viable cells. Optical density was measured at 570 nm using a microplate reader. Each plate contained multiple wells at a given experimental condition and multiple control wells. This procedure was replicated for 2-4 plates per condition [3]. |
| 动物实验 | Male Sprague–Dawley rats (8 weeks old, 180 – 230 g) were used to establish a pressure-overload model as described previously. All animals were separated into four groups (10 rats per group): (i) vehicle-treated sham group; (ii) MG132-treated sham group; (iii) vehicle-treated abdominal aortic banding (AAB) group; and (iv) MG132-treated AAB group. Under intraperitoneal pentobarbital (50 mg/kg) anesthesia, AAB was created using a 5-0 suture tied twice around the abdominal aorta in which. a 21-gauge needle was inserted. The needle was then retracted yielding a 70 – 80% constriction with an outer aortic diameter of 0.8 mm. In the sham surgery rats, the same surgery was performed as described above except the aorta was constricted. At Day 3 after the surgery, MG132-treated rats were intraperitoneally injected with 0.1 mg/kg/day of MG132 for 8 weeks. All control animals were injected with a corresponding volume of vehicle only (0.1% DMSO) [4]. Sixteen-week-old male CD1 mice were used for all our experiments. Thirty minutes before the immobilization procedure, 0.1 mg/kg of buprenorphine was administrated IP. The mice were then anesthetized using isoflurane. The right hindlimb was immobilized as previously described. Briefly, the hindlimb was immobilized 7 days by stapling the foot exploiting normal dorso-tibial flexion using an Autosuture Royal 35W skin stapler. One tine was inserted close to the toe at the plantar portion of the foot while the other was inserted in the distal portion of the gastrocnemius. The other hindlimb was used as a control. During the immobilization period, the mice were injected subcutaneously with MG132 (7.5 mg/kg/dose) or vehicle (DMSO) twice daily. DMSO containing or not MG132 was diluted in sterile pure corn oil (1:100, injected volume 150 μL). After 7 days, the tibialis anterior (TA) muscles of immobilized and non-i |
| 别名 | Z-LLL-al, Z-Leu-Leu-Leu-CHO |
| 化合物与蛋白结合的复合物 |
Crystal structure of MG-132 covalently bound to the main protease (3CLpro/Mpro) of SARS-CoV-2. |
| 分子量 | 475.62 |
| 分子式 | C26H41N3O5 |
| CAS No. | 133407-82-6 |
Powder: -20°C for 3 years | In solvent: -80°C for 1 year
H2O: Insoluble
Ethanol: 47.5 mg/mL (100 mM)
DMSO: 90 mg/mL (189.23 mM)
| 可选溶剂 | 浓度 体积 质量 | 1 mg | 5 mg | 10 mg | 25 mg |
| Ethanol / DMSO | 1 mM | 2.1025 mL | 10.5126 mL | 21.0252 mL | 52.563 mL |
| 5 mM | 0.4205 mL | 2.1025 mL | 4.205 mL | 10.5126 mL | |
| 10 mM | 0.2103 mL | 1.0513 mL | 2.1025 mL | 5.2563 mL | |
| 20 mM | 0.1051 mL | 0.5256 mL | 1.0513 mL | 2.6281 mL | |
| 50 mM | 0.0421 mL | 0.2103 mL | 0.4205 mL | 1.0513 mL | |
| 100 mM | 0.021 mL | 0.1051 mL | 0.2103 mL | 0.5256 mL |
对于不同动物的给药剂量换算,您也可以参考 更多...
请在以下方框中输入您的动物实验信息后点击计算,可以得到母液配置方法和体内配方的制备方法: 比如您的给药剂量是10 mg/kg,每只动物体重20 g,给药体积100 μL,一共给药动物10 只,您使用的配方为5% DMSO+30% PEG300+5% Tween 80+60% ddH2O。那么您的工作液浓度为2 mg/mL。
母液配置方法:2 mg 药物溶于 50 μL DMSO (母液浓度为 40 mg/mL), 如您需要配置的浓度超过该产品的溶解度,请先与我们联系。
体内配方的制备方法:取 50 μL DMSO 主液,加入 300 μL PEG300, 混匀澄清,再加 50 μL Tween 80,混匀澄清,再加 600 μL ddH2O, 混匀澄清。
您可能有的问题的答案可以在抑制剂处理说明中找到,包括如何准备库存溶液,如何存储产品,以及基于细胞的分析和动物实验需要特别注意的问题。
MG-132 133407-82-6 Apoptosis Autophagy Proteases/Proteasome Ubiquitination Proteasome Inhibitor MG 132 complex peptide calpain MG132 Z-LLL-al proteolytic aldehyde inhibit Z-Leu-Leu-Leu-CHO 26S inhibitor
